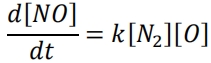EMS604U “Combustion Reactions/Emissions” PBL Questions
(for Week 12; Question numbers continued from Week 10)
化学作业代写 Calculate the equilibrium constant and backward reaction rate if at equilibrium [𝐶𝑂2 ] = 0.20 mol/l, [𝑂2 ] = 0.10 mol/l and [𝐶] = 0.10 mol/l.
11.The decomposition of nitrogen dioxide is given by  The reaction rate constant has the following values:
The reaction rate constant has the following values:
Find:
-the order of this reaction
-the decomposition rate of NO2 at 592 K if its concentration is 0.003 mol/l
-the activation energy
12. Nitrogen oxides (NOx, including NO and NO2) can be formed in combustion by reaction between the nitrogen and oxygen in the air. 化学作业代写
Nitrogen monoxide (or nitric oxide), NO, is produced during the combustion of a stoichiometric fuel-air mixture. The initial rate of formation of nitric oxide may be described by the second-order reaction:
The rate constant, k, is given by an Arrhenius-type expression where the activation energy Ea = 316 kJ/mol, the pre-exponential constant A = 152×106 m3 /mol/s and the universal gas constant R = 8.314 J/mol/K.
At equilibrium when T = 2750 K and P = 5.5 MPa, the mole fractions are:
X(N2) = 0.73, X(O) = 0.5×10-3 and X(NO) = 4×10-3 .
(i) Find the rate of formation of NO in the mixture.
(ii) Find the time for the nitric oxide concentration to reach equilibrium.
13.An elementary reaction in a complex combustion process can be written as
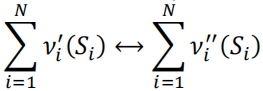
(i)Write an expression for the forward, backward and net reaction rates with respect to species 𝑆𝑖 .
(ii)Considering the simple case of one species only present in the reactant side of the forward equation, find an expression for the concentration time behaviour ([𝑆𝑖 ] expression as a function of time), in the case of an overall first order reaction. Plot the corresponding typical graph.
14.(i) The equilibrium constant for a reaction involving species 𝑆𝑖 , 𝑖 = 1, … ,6 is given by  write the chemical equation for the reaction.
write the chemical equation for the reaction.
(ii) The forward (forming CO2) and backward reaction rates of the following reaction are 𝑘𝑓 = 0.2 l/mol/s and 𝑘𝑏 respectively.
𝐶 + 𝑂2 ⟷ 𝐶𝑂2
Calculate the equilibrium constant and backward reaction rate if at equilibrium [𝐶𝑂2 ] = 0.20 mol/l, [𝑂2 ] = 0.10 mol/l and [𝐶] = 0.10 mol/l.

15.Hydrogen is burned with air under stoichiometric conditions in an internal combustion engine. 化学作业代写
After ignition has taken place, the reacting mixture is assumed to consist of hydrogen, oxygen, water vapour and nitrogen as ideal gases. Analysis of the combustion products at equilibrium shows that the mole fraction of hydrogen fuel is 0.022 and that the pressure at the sampling point is 25P0, where P0 is the reference pressure.
(i)Find the degree of reaction ε.
(ii)Calculate the equilibrium constant Kp.
16.In consecutive (or series) reactions, the products of a reaction undergo further reaction to give other products. A simple example can be given as
In the above equation, 𝑘1 and 𝑘2 are the consecutive reaction rate constants.
(i)Derive a formula to calculate the net molar rate of production of the intermediate product C.
(ii)What is the ratio of the rate constants when the reaction is in equilibrium with respect to C?
17.An overall combustion process can be assumed to follow the procedure given as 化学作业代写
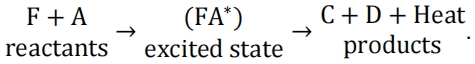
Experiments found that there is an energy barrier to be overcome for the reaction to happen, which is the activation energy for the forward reaction leading to the formation of the activated complex (FA)*. There is also an energy associated to the energy released from (FA)* to split into species C+D, involving the heat released from the exothermic reaction. Draw a diagram of energy versus time, indicating the relevant forms of energy including the net amount of heat released during the combustion with brief explanations.
更多代写:留学生网课代考 gre网考作弊 北美代写网课代考 美国Essay代写费用 论文查重免费 宏观经济学原理代考
合作平台:essay代写 论文代写 写手招聘 英国留学生代写