General Chemistry Lecture I (Chem 001-01) Exam 3
Monday, November 19, 2018
Chemistry化学代考 solids consist of atoms or molecules held together by dipole-dipole forces, London dispersion forces, and/or hydrogen
Please show all your work.
Try to make your flow of logic neat and obvious. There are a total of 100 points in this exam.
Name (please print):
My signature signifies that I have neither given nor received help on this exam.
Honor Pledge (please sign):
ΔCP = Σ n ΔCP,m products – Σ n ΔCP,m reactants
ΔHº(T2) = ΔHº(T1) + (T2 – T1)
ΔCP Density of a unit cell = [(molar mass)(1/NA)(#lattice points/unit cell)]/(length of edge)3
ΔHºrxn = Σ Bond enthalpies of bonds broken – Σ Bond Enthalpies of bonds formed
Topic I: Intermolecular Forces 10% Chemistry化学代考
1.If a solid line represents a covalent bond and a dotted line represents intermolecularattraction, which of these choices shows a hydrogen bond? Circle all that apply. (2.5 points)
a) —N·······H—O—
b) H—H·······H—H
c) —O·······H—O—
d) —C·······H—F—
e) —O·······H—C—
2.a) What type of intermolecular force is used to explain the observed trend in boiling points for the Group 4A molecules shown in the figure below? (5points)
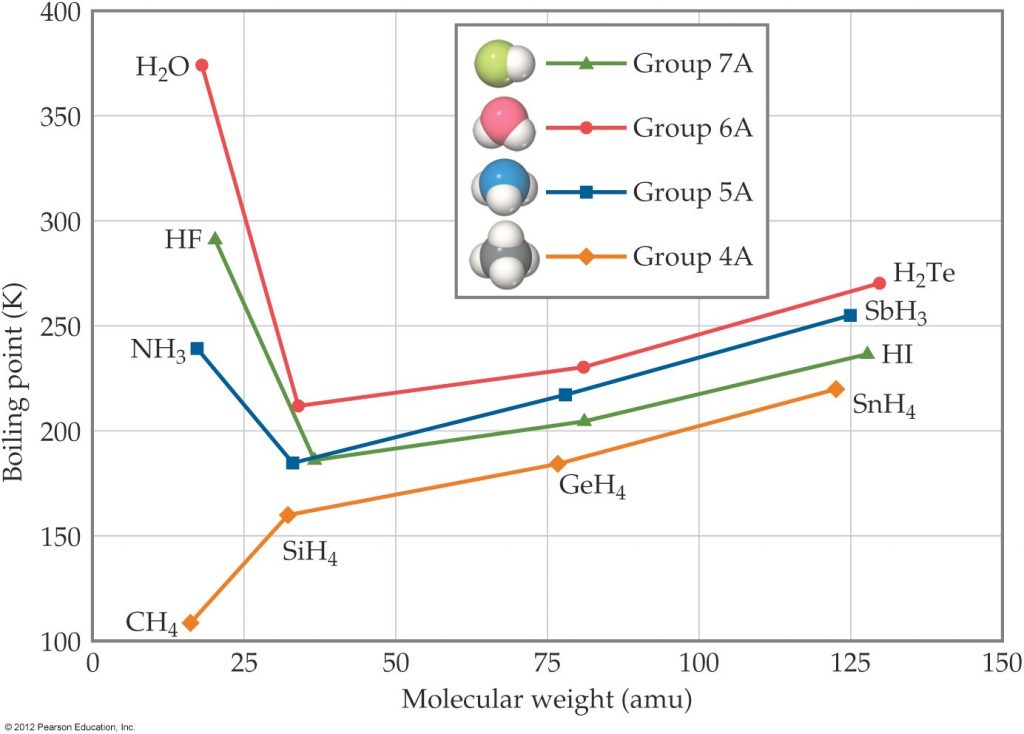
b)Why is the boiling point of ammonia, NH3, higher than the boiling point of PH3? (5points)
c)Why is the boiling point of PH3higher than the boiling point of SiH4? (5 points)

Topic II: Solid State 40% Chemistry化学代考
3.(8points)
a)solids consist of atoms or molecules held together by dipole-dipole forces, London dispersion forces, and/or hydrogen
A)Ionic
B)Molecular
C)Metallic
D)Covalent-network
E)Metallic andcovalent-network
b)Potassium metal crystallizes in a body-centered cubic structure with a unit cell edge lengthof 5.31 Å. What is the radius of a potassium atom in Å?
4.(20points)
a)The compound CuCl has the zinc blende, ZnS, structure and a density of 3.41 g/cm3. In the zinc blende structure sulfides are on fcc lattice points and zinc cations are in half of the tetrahedral pockets. What is the length of an edge of the unitcell? Chemistry化学代考
b)What is the coordination number of Cu+in the zinc blende structure? (i.e. how many chlorides surround each Cu+)
5.(12 points) NaCl crystallizes in a face-centered cubic cell.
a)What is the total number of ions (Na+ ions and Cl- ions) that lie within a unit cell ofNaCl?
b)The length of the edge of a unit cell of NaCl is 564 pm. What is the distance between Na+and Cl– in NaCl?
c)Is this bond length, determined in part c, the same throughout the structure ofNaCl?
Topic III: The First Law, Internal Energy, Heat, Work, Enthalpy, Phase Changes 25% Chemistry化学代考
6.(20points)
a)A gas, while expanding, absorbs 1250 J of heat and does 2.43 kJ of work. What is ΔU for the gas?
b)What is the value of w for an ideal gas expanding reversibly and isothermally if 2.00 molat 300.0 K and 3.00 atm expands from 6.00L to 18.0 L to a final pressure of 1.20 atm?
c)765 J of heat is transferred at constant pressure to 1.00 mole of Kr(g)at 298 K and 1.00 atm. The same amount of heat is added to 1.00 mole of CO2(g) under the same conditions. Which gas, CO2 or Kr, has a higher temperature assuming they behave as ideal gases? Chemistry化学代考
d)Calculate the heat that must be supplied to a copper kettle of mass 400.0 g containing 300.0 g of water to raise its temperature from 20.0 °C to the boiling point of water, 100.0°
The specific heat of copper is 0.385 J/g °C and the specific heat of water is 4.184 J/g°C.
7.(5points)
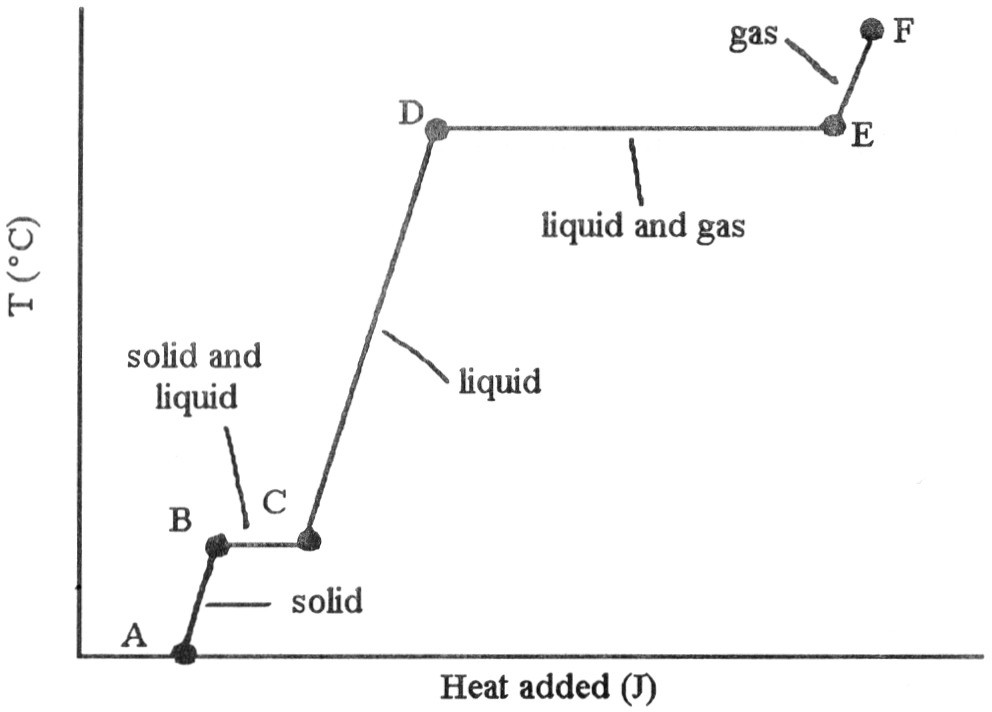
a)On the heating curve shown below, label the melting point and the boiling point for the substance.
b)Ofthe following, is an exothermic process.
a)melting
b)subliming
c)freezing
d)boiling
e)All of the above are exothermic.
Topic IV: Enthalpies of Reaction 25% Chemistry化学代考
8.(10points)
When PCl3(g) is formed from white phosphorus and chlorine gas, ΔHº for the reaction is -306 kJ/mol, while for the formation of PCl3(g) from red phosphorus (an allotrope of white phosphorus) and chlorine gas, ΔHº is -288 kJ/mol. From this information calculate ΔHº for the conversion of red phosphorus to white phosphorus.
PRed → PWhite ΔHº = ?
9.(15points)
a)Write the balanced chemical equations that correspond to the following data at standard conditions:
ΔH°combustion: C5H12(g) = -3537 kJ/mol
ΔH°formation: CO2(g) = – 393.5 kJ/mol
ΔH°formation: H2O(l) = – 285.8 kJ/mol
b)Use the data in part a to determine the ΔH°ffor C5H12(g).
更多代写:C++作业代做 duolingo代考 Econ网课代上推荐 Essay代写网站 Dissertation学位论文代写 本科essay查重率
合作平台:essay代写 论文代写 写手招聘 英国留学生代写